Abstract
The most transformative approach for controlling chronic Graft-versus-Host Disease (cGvHD) after allogeniec hematopoietic cell transplantation (alloHCT) would be the prevention of its most severe and irreversible clinical manifestations instead of treating already established disease. Belimumab is a monoclonal antibody, approved for treatment of systemic lupus erythematosus and active lupus nephritis, which inhibits binding of B-cell-activating factor (BAFF) to its receptors on B cells, thus inhibiting the survival of autoreactive B cells. Given the role of B cells in cGvHD pathophysiology and the now substantiated role of BAFF in promoting B Cell Receptor signaling in cGvHD (Jia W et al Blood 2021), belimumab might have a role in prevention of cGvHD. We hypothesized that targeting BAFF early after alloHCT would be well-tolerated and have a favorable effect on the incidence and severity of cGvHD.
We are presenting data on the first 8/10 patients (Pts) enrolled in the single-center, investigator-initiated phase-1 trial. Belimumab was administered i.v. at 10 mg/kg every 2 weeks for 3 doses followed by 4 doses at monthly intervals, for a total of 7 doses, starting 50-80 days after alloHCT. All Pts were adults who received mobilized peripheral blood grafts from fully matched related or unrelated donors after myeloablative or non-myeloablative conditioning. GvHD prophylaxis was with tacrolimus and methotrexate; one Pt received post-transplant cyclophosphamide. All Pts were in complete remission on day +30 after alloHCT. Pts who received ≥1 dose of belimumab were evaluable for safety and ≥2 doses for efficacy assessment. cGvHD was diagnosed according to the NIH criteria. All analyses are descriptive. Specimens for correlative studies are cryopreserved until the study end.
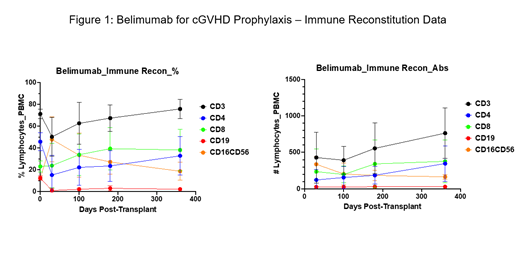
We found that Pts tolerated belimumab well, with no reported grade ≥3 treatment-related adverse events. There were no significant infections or myelosuppression. Seven out of eight Pts on the study received all 7 doses of belimumab, as planned. Of those 7 Pts, 5 Pts are without any evidence of cGvHD and completely off immunosuppression at the median of 18 months (mo) after completing belimumab (median of 24 mo after alloHCT). Seven mo after completing belimumab on study, 1 Pt developed cGvHD involving skin, eyes and liver, and died from complications of pneumonia and liver failure due to longstanding hemochromatosis, iron overload and possible liver GvHD. Another Pt relapsed with leukemia 1 mo after completing belimumab. He is now in remission after treatment with enasidenib and 2 donor lymphocyte infusions (DLI), 12 mo after completing belimumab (18 mo after alloHCT); he developed mild eye GvHD following DLI. One out of eight Pts on the study stopped treatment early, after receiving only 3 doses, due to thrombocytopenia (unrelated to belimumab) and insurance issues. He was found to have relapsed lymphoma 3 mo later, was treated with venetoclax and DLI, and remains in remission with no evidence of cGvHD 15 mo after stopping belimumab. Two additional patients are being enrolled. Immune reconstitution studies are showing that B-cell counts remain low 1 year out of transplant in all Pts (Figure 1).
This preliminary data describes for the first time the use of belimumab for prophylaxis of cGvHD. Overall, belimumab was very well tolerated. Only 1 Pt on the study developed cGvHD. It is worth noting that his cGvHD developed 7 mo after completing belimumab suggesting that a longer duration of therapy should be tested in future studies. The 2 Pts with relapsed disease both had high risk malignancies. Risk stratification of high risk patients will be critical in future prospective studies. While more Pts are needed to further assess the impact of prophylactic belimumab on the incidence of cGVHD, these preliminary results are encouraging. Absence of severe infections is reassuring. B-cell reconstitution was delayed, as expected. Ongoing correlative studies will further evaluate BAFF levels and B-cell subset reconstitution after alloHCT.
Pusic: Syndax: Other: Advisory Board. Sarantopoulos: Rigel: Other: Advisory Board. Uy: Macrogenics: Research Funding; Jazz: Consultancy; Genentech: Consultancy; Astellas: Honoraria, Speakers Bureau; Novartis: Consultancy; AbbVie: Consultancy; GlaxoSmithKline: Consultancy; Agios: Consultancy.
Drug: Belimumab This study: Belimumab for prophylaxis of chronic GvHD. Approved indication: Belimumab is approved for treatment of systemic lupus erythematosus and active lupus nephritis.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal